
Chromatography: All You Need To Know About It
Chromatography is an exciting technique that is used to separate different components of a sample. It plays a critical role in scientific research, medicine, Environmental management, and other sectors. In this guide, you will learn chromatography, how it is carried out, the different types, and their significance. We shall also examine the uses and ways it affects our daily lives.
What is Chromatography?
Chromatography is a method by which components of a mixture can be separated by the speed at which they can move through a stationary phase. The term chromatography itself has a Greek origin, with the word chromat meaning color and the word graphein meaning to write. While the initial application of chromatography was based on color separation, it is currently applicable for separating mixtures of gases, liquids, and even biological molecules.
How Does Chromatography Work?
The Basic Principle
Mobile Phase: This material flows and serves as a vehicle for the mixture. It can be in a liquid state or gaseous form.
Stationary Phase: This material remains static and comes into contact with parts of the solution. It can be a solid or a liquid phase on a solid phase.
The sample to be analyzed is first injected into the mobile phase of the system. As the mobile phase passes through the stationary phase, the various parts of the mixture make the journey at different rates. Because of the difference in speed, these components end up separating. The time a component takes to spend in the system is known as its retention time.
Example
Consider a solution containing some colored dyes, and you put a drop of this solution on a piece of paper (the stationary phase). If you dip the paper into water (the mobile phase), the water will move up the paper, carrying the dyes. Different dyes will travel at different speeds, causing them to separate into distinct bands. This simple example illustrates the fundamental principle of chromatography.
Types of Chromatography
Chromatography can be categorized into several categories depending on the type of mixture or application.
1. Paper Chromatography
Among all chromatographic techniques, paper chromatography is one of the simplest forms of chromatography. One of them uses a sheet of paper as the stationary phase. A small drop of the mixture is placed at the base of the paper, and the paper is then placed in a solvent (the mobile phase). When the solvent is pulled through the paper with capillary actions, these components of the mixture move along with the solvent. Parts of it circulate at various speeds to make different lines and shades on the paper. They are widely used in teaching laboratories because they are easy to prepare and inexpensive.
2. Thin-Layer Chromatography (TLC)

img credit: dreamstime
Thin-layer chromatography is similar to paper chromatography. However, the solvent moves through a thin material layer, such as a bare glass, plastic, or metal plate coated with a thin silica gel or alumina layer. It is used as a small spot on the plate; the plate is then placed in a solvent container. The process continues as the solvent climbs the plate, taking with it the various components of the mixture. Compared to paper chromatography, TLC is faster and yields more separation among the components. It is commonly used for observing the rate of chemical reactions and testing the level of compound purity.
3. Liquid Chromatography
Liquid chromatography involves flowing the liquid containing the mixture ( the mobile phase) through a column containing a stationary phase in the form of a solid. These components of the mixture have different affinities to the stationary phase, and hence, they progress in the column in different ways.
High-Performance Liquid Chromatography (HPLC): This technique employs the application of high pressure as a means of forcing the mobile phase through a tightly packed column. HPLC provides high resolution and is used for separating and analyzing complex mixtures, such as pharmaceuticals and biological samples.
Normal-Phase Chromatography: It includes a stationary phase that is polar in nature and a mobile phase that is non-polar in nature. In this method, the components are separated depending on their polarity.
Reverse-Phase Chromatography: The stationary phase is not soluble in polar solvents, while the mobile phase is soluble in polar solvents. This is the type of liquid chromatography applied in various applications; it is the most common.
4. Gas Chromatography
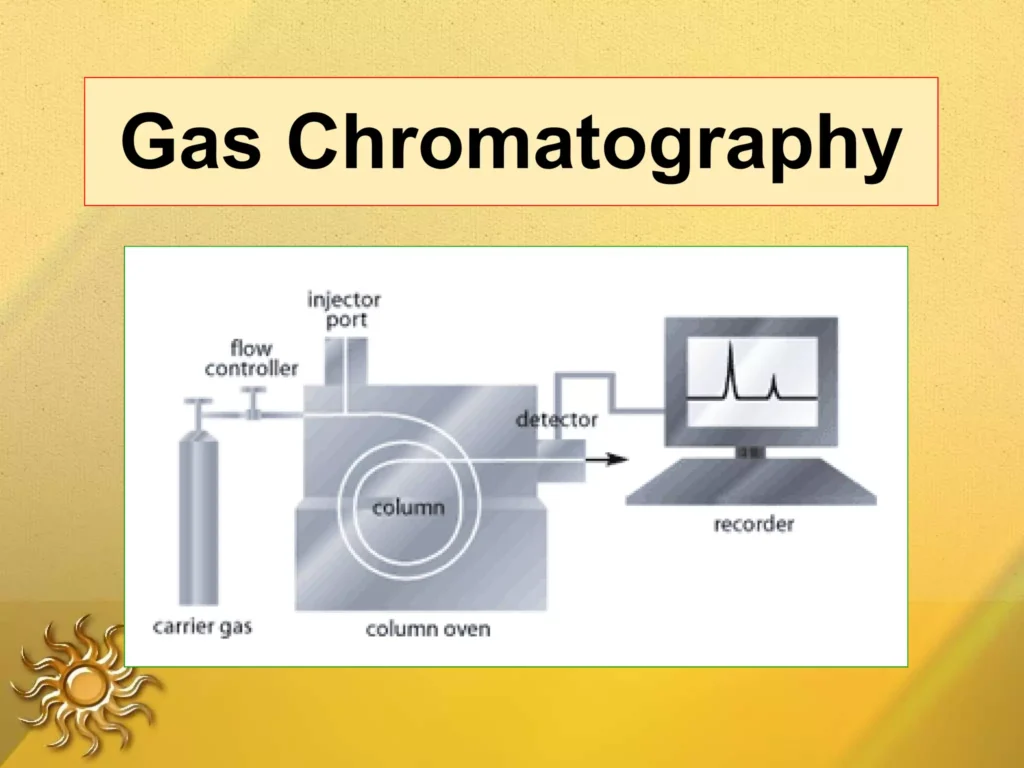
img credit: slideshare
The analysis of compounds that can be vaporized without breaking down is done by a method known as gas chromatography. The mobile phase can be an inert gas like helium or nitrogen, and the stationary phase can be a layer of liquid or polymer on an inert solid matrix within the column. The sample is taken in the vapor phase and introduced into the column, where it comes in contact with the stationary phase. Some components take longer to go through this process, allowing them to be separated and perhaps identified. The application of gas chromatography is used in environmental analysis, forensic applications and the petrochemical sector.
5. Ion Exchange Chromatography
Ion exchange chromatography is the process in which the molecules are separated by electric charge. The stationary phase has charged groups that attract the mobile phase with an opposite charge.
Cation Exchange: This makes the stationary phase negatively charged. Hence, it analytically retains only the positively charged ions known as cations.
Anion Exchange: In this case, the stationary phase is of a positive charge to attract the negative ions or anions.
This method can be especially valuable for separating proteins, peptides, and nucleotides.
Wrapping Up
Chromatography serves as a versatile laboratory technique for separating compounds in various fields. It is thoroughly used in Chemistry and is involved in various steps. Chromatography has been widely used in the purification of chemicals, especially when synthesizing new chemicals. It can be used to identify substances of unknown nature based on their retention time or any other factor related to the substance. Chromatography is applied to observe the reaction’s course and find the time when the reaction occurred. So, now, you know how to use Chromatography and when you can apply it!

0 comments